View Winners →
View Winners → 
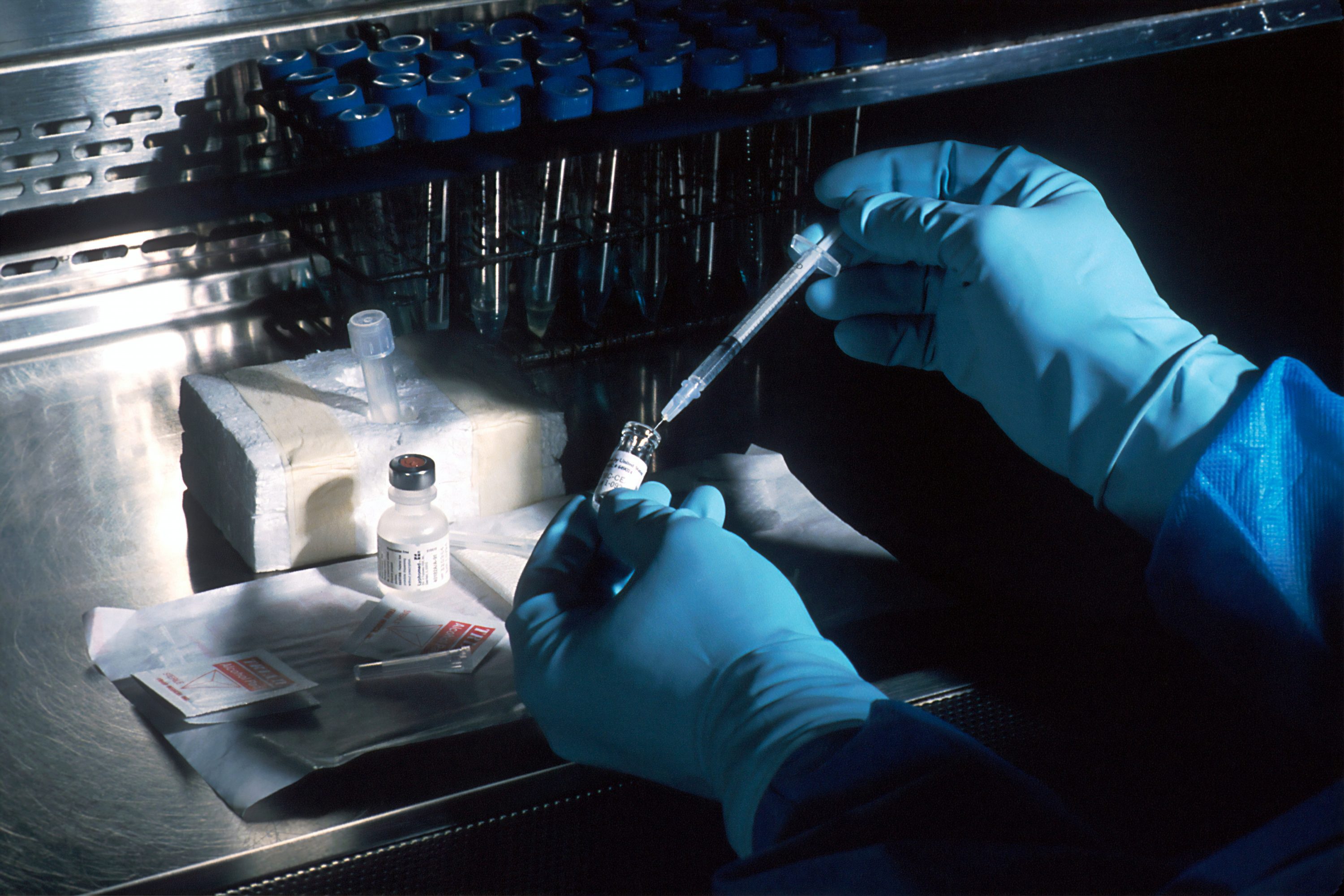
K. Barry Sharpless from Scripps Research in La Jolla, along with Carolyn R. Bertozzi from Stanford University and Morten Meldal from the University of Copenhagen, have won the Nobel Prize in chemistry for the development of click chemistry and bioorthogonal chemistry, the Royal Swedish Academy of Sciences announced Wednesday.
It is the second time Sharpless has won a Nobel Prize. He is a W. M. Keck professor at Scripps Research.
Sharpless and Meldal set the foundation for a functional form of chemistry — click chemistry — in which molecular building blocks snap together quickly and efficiently. Bertozzi took click chemistry to a new dimension and started using it in living organisms.
“This year’s Prize in Chemistry deals with not overcomplicating matters, instead working with what is easy and simple. Functional molecules can be built even by taking a straightforward route,” John Aqvist, Chair of the Nobel Committee for Chemistry, said in a statement.
Sharpless coined the concept of click chemistry around 2000. It was a form of simple and reliable chemistry that resulted in reactions occurring quickly and unwanted by-products were avoided.
Sharpless and Meldal, independent of each other, presented what is described as the crown jewel of click chemistry: the copper catalyzed azide-alkyne cycloaddition. It is a chemical reaction that is now in widespread use, particularly in the development of pharmaceuticals, for mapping DNA and creating materials that are more fit for purpose.
Bertozzi expanded the use of click chemistry by using it to map biomolecules on the surface of cells — glycans — and developed click reactions that work inside living organisms.
Her bioorthogonal reactions take place without disrupting the normal chemistry of the cell. Using bioorthogonal reactions, researchers have improved the targeting of cancer pharmaceuticals, which are now being tested in clinical trials.