View Winners →
View Winners → L.A. County Unveils COVID-19 Vaccine Distribution Plan
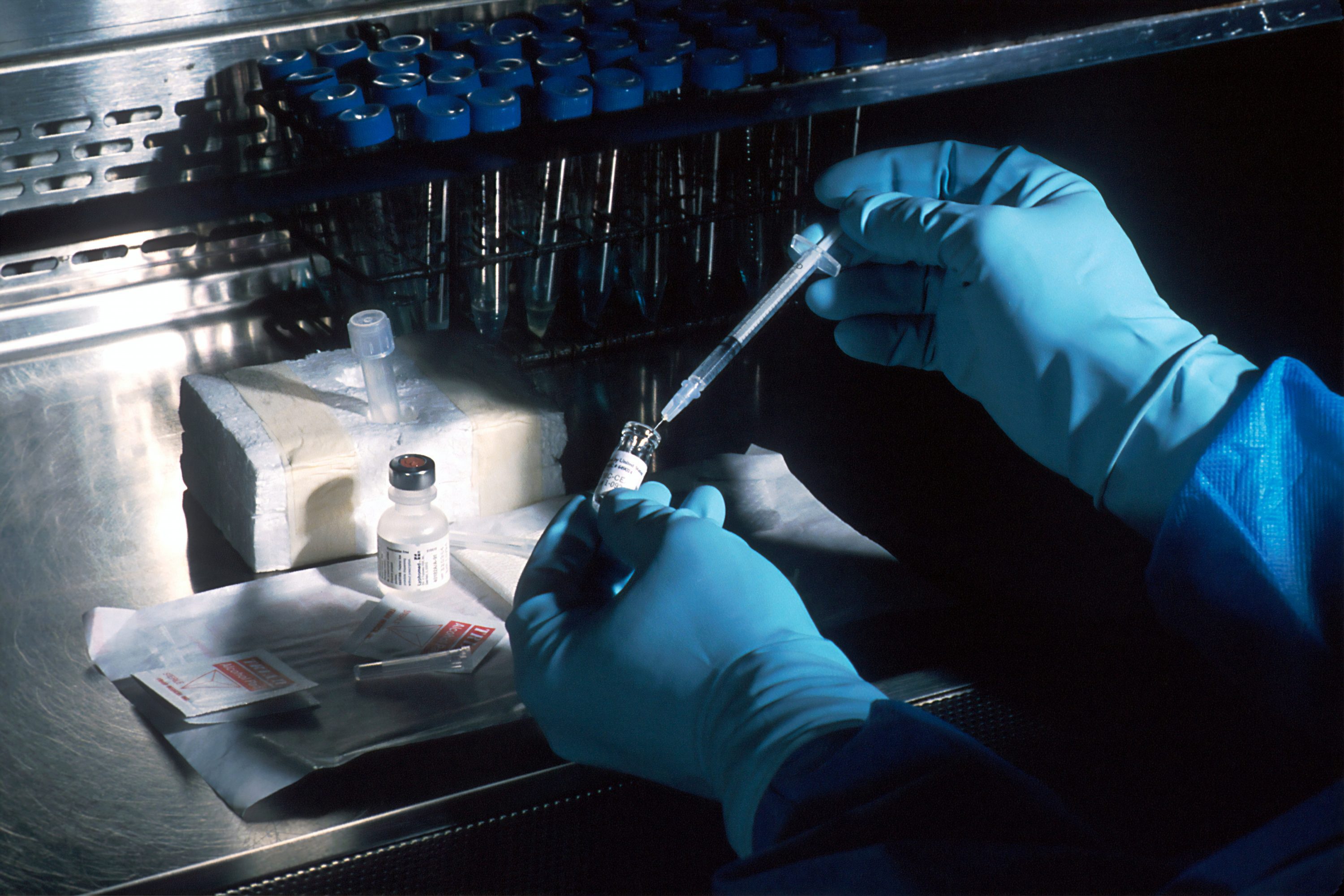
Image used for illustration only. It does not depict the actual vaccine. | Courtesy photo by Daniel Schludi on Unsplash
With the Food and Drug Administration (F.D.A.) expected to issue an emergency use authorization for Pfizer/BioNTech’s COVID-19 vaccine, Los Angeles County public health officials have announced their plan to distribute initial allotments of the immunization.
The county is looking at a complex logistical process to vaccinate residents, some of which have expressed hesitancy. A survey from the Associated Press-NORC Center for Public Affairs Research published earlier this month showed that only 47% of Americans plan to get the vaccine when it becomes available, while 26% do not plant to be inoculated and 27% are unsure.
“As we implement the county plan to vaccinate literally millions of Angelenos, it will be critically important that we address this vaccine hesitancy with accurate, understandable, culturally sensitive, and linguistically appropriate information — relying on trusted community leaders to help deliver these messages,” Dr. Paul Simon, chief science officer for the L.A County Department of Public Health, said during a press briefing Thursday afternoon.
According to the survey, many Americans have concerns about the vaccine’s safety and its equitable distribution.
Addressing these concerns, Dr. Simon assured residents that the vaccine, which has a 95% efficacy rate, is safe and has so far not been shown to cause severe adverse reactions. However, he did point out that some clinical study participants did experience symptoms such as soreness near the vaccine site, headache, muscle ache, fever, chills, and fatigue.
However, Dr. June Raine, chief executive of the UK’s Medicines and Healthcare products Regulatory Agency, on Wednesday warned against administering the Pfizer vaccine to anyone “with a history of immediate-onset anaphylaxis to a vaccine, medicine or food” after two health care workers had adverse reactions to the shot. Anaphylaxis is a rare, though potentially life-threatening, allergic reaction which can cause hypotension, constriction of the airways, a weak and rapid pulse, nausea, vomiting, diarrhea, a skin rash, dizziness or fainting, according to the Mayo Clinic. The two health care workers received treatment and are now recovering.
F.D.A. officials on Thursday said, “that because of the British cases they would require Pfizer to increase its monitoring for anaphylaxis and submit data on it once the vaccine comes into use,” according to The New York Times.
As to equitable distribution of the vaccine, Dr. Simon said that not even elected officials will receive special treatment.
“Equity is a fundamental principal here,” he said. “We wanna make sure all people have access and that those that are at greatest risk, either because of higher risk of exposure or greater risk of severe illness because of chronic health conditions or other factors, have more immediate access to the vaccine.”
Supply of the vaccine will be limited at first. California expects to receive 327,000 doses in the initial distribution sometime early next week, pending authorization of the vaccine by the F.D.A., and L.A. County will receive 82,875 of those doses. County officials expect to receive an additional allocation of approximately 250,000 doses following the first shipment and then another 150,000 the week after that, according to Simon.
The Pfizer vaccine requires two doses to be effective and that second round of immunizations should be distributed in early January, at which time the county hopes to be receiving weekly allotments.
Initial allocations will be sent to nine sites with ultra-cold freezers (a requirement for the Pfizer vaccine) and then distributed to 83 acute care hospitals across the county to be administered to health care workers prioritized based on risk.
In this first part of the phased rollout, the focus will be on vaccinating health care workers and residents and staff of long-term care facilities, in alignment with the recommendations of the Centers for Disease Control and Prevention’s (C.D.C.). Long-term care facility residents and staff will receive the vaccine from CVS and Walgreen pharmacies, through a federal partnership program.
Additional phases of vaccination distribution will focus on essential workers and high-risk groups, including seniors and those with chronic health conditions. Plans are being developed for how individuals in these broad groups will be prioritized and how the vaccines will be delivered to them. Planning for these phases is occurring in coordination with the C.D.C., state health department, the local health care community, and many other entities.
The county also needs to track which individuals have been vaccinated and if the Moderna vaccine is approved, they will need to ensure that individuals receive the second dose from the same provider. Data systems are reportedly being established to track this information.
“When someone receives their first vaccination, they will receive a yellow card with that vaccination, the date, and the type, and when they need to come back,” Simon said. Information logged in the data system will be tracked and reminders for the second dose will be sent through text or email.
Over time, as more vaccine is available it will be offered to everyone. This will likely take months and may not be widely available to the general public until spring or summer of 2021, according to public health officials.
Dr. Simon believes the county is nowhere near close to going back to normal, especially as daily COVID-19 cases and hospitalizations keep breaking previous records. On Friday, L.A. County entered what officials called “uncharted territory” as 13,815 new coronavirus cases were recorded, the Los Angeles Times reported.
“I am very concerned. I’ve worked in public health for 30-plus years; I’ve never been more concerned than I am right now” Simon explained Thursday. “We’re not even close to being able to move back out.”
Even after the vaccine is administered, questions about how long protection lasts and the degree to which the vaccine prevents asymptomatic infection still need to be answered. “I think there’s still a lot of questions about how long this vaccine works. You know we’re hopeful it will lead to long-term protection, but again that remains to be seen,” cautioned Simon.
Of course, all these plans are dependent on whether the F.D.A. issues an emergency use authorization for Pfizer’s vaccine. On Thursday, an advisory panel comprised of independent scientific experts recommended that the F.D.A. authorize the vaccine for individuals 16 and older. Originally, the F.D.A. was expected to grant the authorization on Saturday but on Friday the Washington Post reported that Mark Meadows, President Trump’s chief of staff, told Commissioner Dr. Stephen M. Hahn to submit his resignation if the vaccine was not approved by Friday, according to a senior administration official.
As of Friday, at 3 p.m., no authorization had yet been issued.