The identification of a small molecule drug that can target the brain’s circadian clock proteins may prove effective for treating glioblastoma, the most common cancerous brain tumor in adults, researchers at the Keck School of Medicine of USC announced Monday.
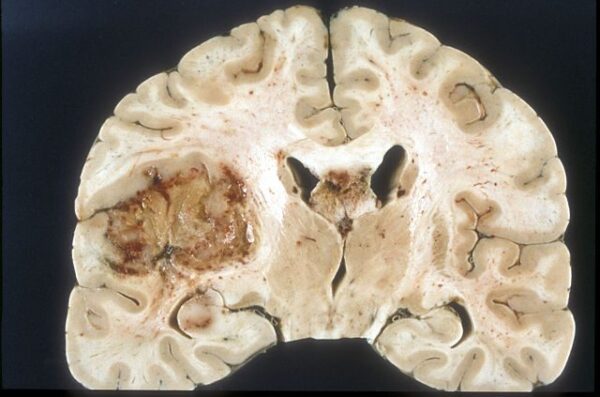
Glioblastoma is an aggressive disease. Patients survive an average of just 15 months once they are diagnosed. Despite more than two decades of research on the causes and treatments of glioblastoma, that prognosis has hardly improved.
But recent work by a Keck School of Medicine team has demonstrated that circadian clock proteins, which help coordinate changes in the body’s functions over the course of a day, may play a key role in glioblastoma growth and proliferation after current standard treatments, according to research published in Proceedings of the National Academy of Sciences.
The discovery has led to a potential breakthrough: the identification of a small molecule drug, known as SHP656, that can target the clock proteins and may prove effective for treating the disease, according to Steve Kay, USC professor of biological sciences at the Keck School of Medicine and director of the USC Michelson Center for Convergent Bioscience.
Kay assembled a collaborative that unites academics with expertise in glioblastoma, circadian clock biology and biological chemistry with Synchronicity Pharma, a biotechnology startup that he co-founded.
“We’re now starting to march down the path of clinical drug development — turning this from a science story into a translational one,” said Kay, the study’s senior author, who also co-directs the USC Norris Brown Center for Cancer Drug Development.
The first symptoms of glioblastoma can include everything from blurred vision, headaches and nausea to seizures and personality changes. Patients typically undergo a brain scan, which identifies the tumor, then receive a combination of surgery, radiation and chemotherapy treatment. While most tumors shrink substantially after the initial treatment, few patients experience sustained remission.
“In the vast majority of patients, the cancer returns,” Kay said. “And when it returns, it*s resistant to chemotherapy and radiation.”
Researchers believe that the cancer returns because a small number of “cancer stem cells” are left behind after surgery, chemotherapy and radiation.
These stem cells can multiply and spread very quickly, and research by Kay’s team helps explain why. He and Dr. Jeremy N. Rich, of the University of Pittsburgh, found that cancer stem cells hijack the body’s circadian clock machinery, allowing them to spread more quickly and resist the effects of chemotherapy and radiation treatment.
With that knowledge, Kay and his collaborators created and tested thousands of molecules capable of binding to — and potentially neutralizing — the rogue circadian clock proteins inside cancer stem cells, according to USC.
Using several advanced techniques, including artificial intelligence, to determine which molecule was best suited to fight glioblastoma, the team studied how each new molecule would bind to the clock proteins, searching for the perfect “lock-and-key” fit, and pinpointed one particularly promising molecule: SHP656, USC said.
The next step was to test the effectiveness of SHP656 against actual cancer cells. Using glioblastoma stem cells collected from patients, the researchers showed that SHP656 reduced the growth of cancer stem cells, but did not harm the body’s normal stem cells, the study found.
“We’re seeing that the molecule acts differently on healthy brain cells versus tumor cells,” Kay said. “This was a real leap forward in our understanding of how we can develop drugs that target clock proteins.”