The Western States Scientific Safety Review Workgroup completed its review of the federal process Saturday night and concluded the Moderna COVID-19 vaccine is safe and efficacious for use in California, Nevada, Oregon and Washington. The group provided its confirmation to the governors of these four western states Sunday morning, making the Moderna vaccine the second COVID-19 vaccine supported for use in these states. Shipments are expected early this week.
The Food and Drug Administration authorized the vaccine, which was developed in part with National Institutes of Health, for emergency use on Friday.
Washington, Oregon and Nevada joined California’s COVID-19 Scientific Safety Review Workgroup in October. The workgroup, made up of scientists with expertise in immunization and public health, has concurrently and independently reviewed the F.D.A.’s actions related to COVID-19 vaccinations. It will continue to evaluate other COVID-19 vaccines as they go through the federal process.
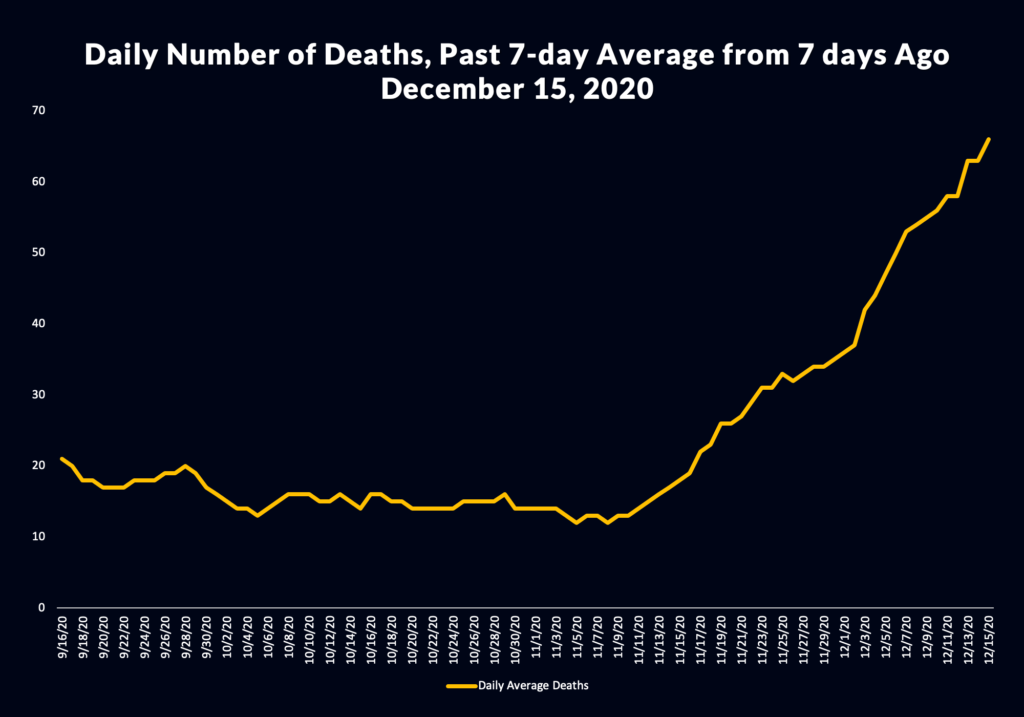
“While California is in some of the darkest days of our COVID-19 surge, with too many families grieving lost loved ones, there is light as more vaccines are approved for distribution,” said Governor Gavin Newsom. “With the Moderna vaccine in circulation, we have another tool to fight this deadly disease.”
In Los Angeles County, health officials last week said the initial allotment from Moderna would be used to vaccinate staff and residents at long-term care facilities and skilled nursing facilities, EMTs and paramedics and those administering vaccines.