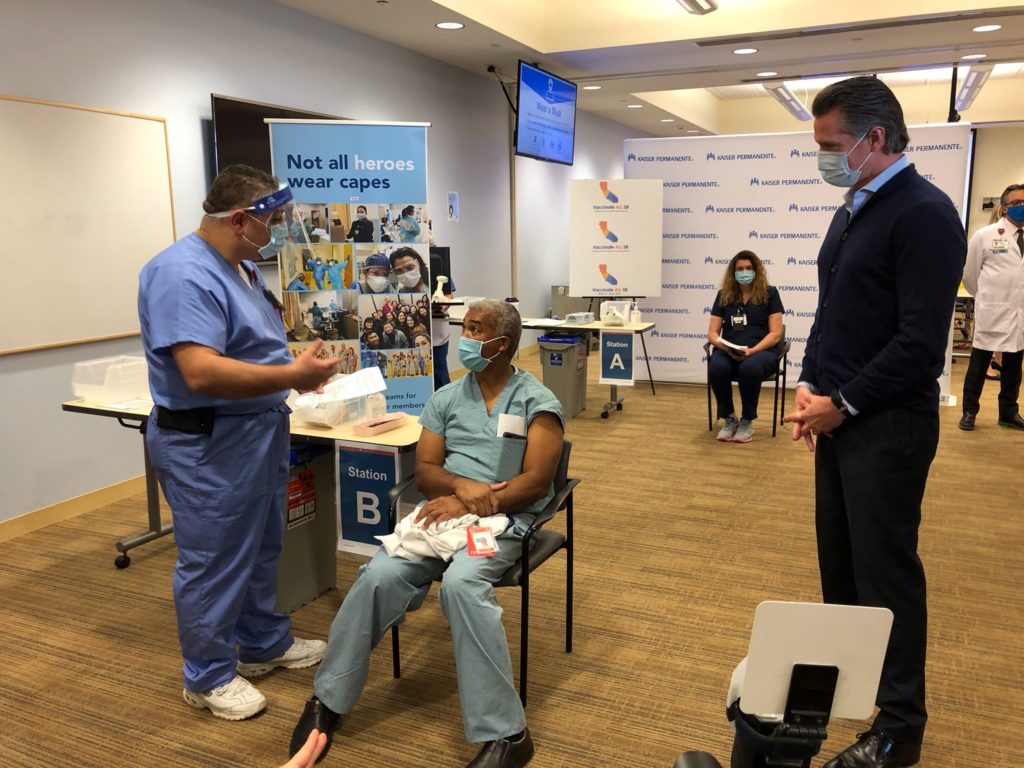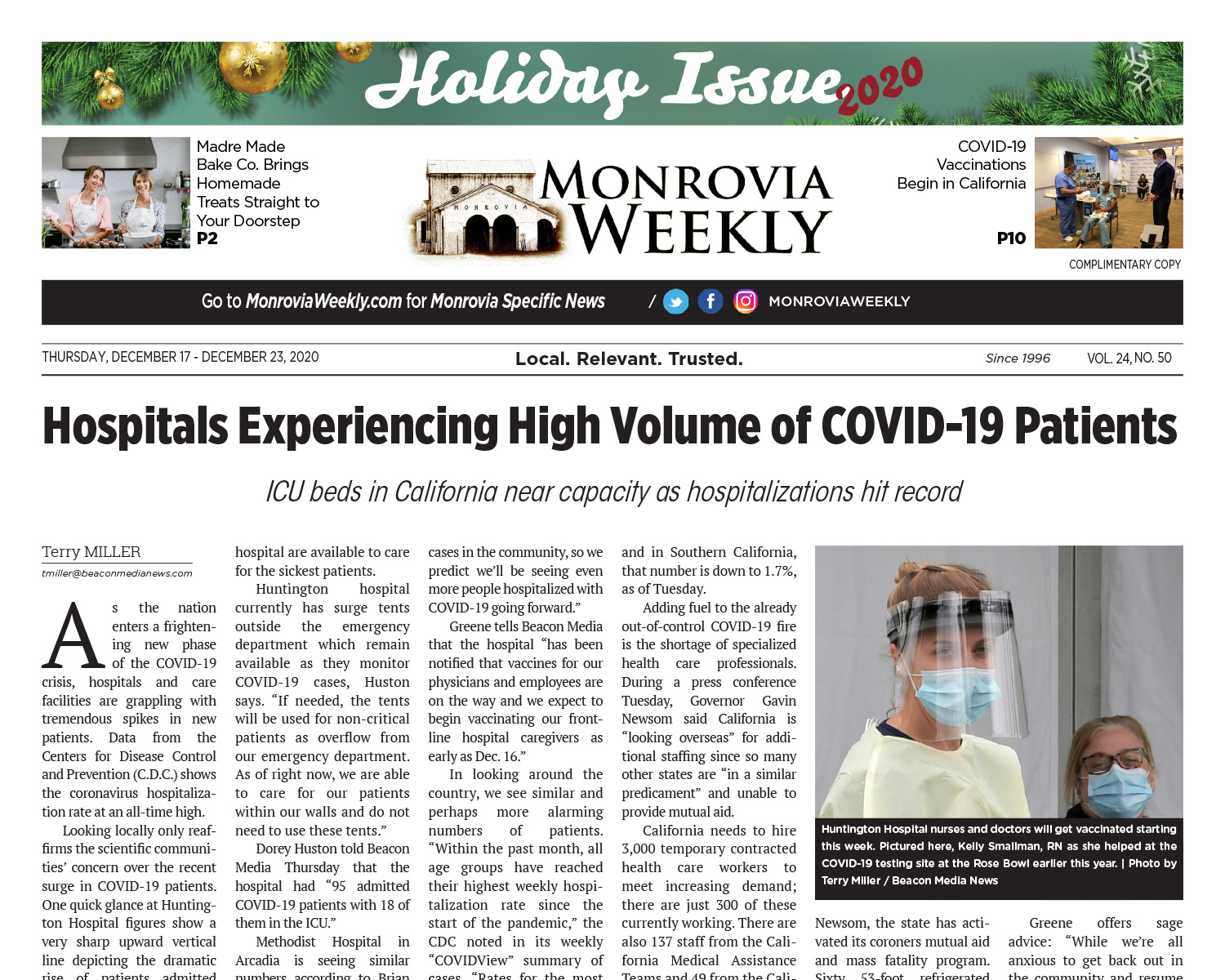
The Western States Scientific Safety Review Workgroup completed their concurrent review of the federal process and on Sunday confirmed the Pfizer COVID-19 vaccine is safe and efficacious. The group provided their confirmation to the governors of California, Nevada, Oregon and Washington Sunday morning.
Amid concern that political pressure would rush the U.S. Food and Drug Administration’s process, Washington, Oregon and Nevada joined California’s COVID-19 Scientific Safety Review Workgroup in October, which has worked concurrently and independently to review the agency’s actions related to COVID-19 vaccinations.
The group of scientists “unanimously” recommends that the Pfzier BioNTech COVID-19 vaccine be used in the four western states. I also concluded that “equity has been considered appropriately in the clinical trials and urges that equity continue to be a guiding principle in immunization implementation, monitoring and communication.”
In a statement, Governor Gavin Newsom said, “With shipments of the vaccine soon on their way to California, we are working hand-in-hand with local public health officials to get the vaccine out to the first phase of recipients.”
In response to concerns over anaphylactic reactions after two health care workers in the UK experience adverse responses to the vaccine last week, the workgroup underscored “the importance — as with any vaccine — of providing the vaccine in locations that are prepared to treat anaphylactic reactions or any other unexpected reactions, as noted in standard guidance from C.D.C.”
The group acknowledged that several unanswered questions about the vaccine remain, “including the duration of vaccine induced protection; the effect of vaccination on asymptomatic infection; the effect of vaccination on transmission of SARS-CoV-2; and the safety and efficacy of the Pfizer BioNTech COVID-19 vaccine in pregnant women and children under 16 years of age.”
The workgroup will continue to evaluate other COVID-19 vaccines after they are routed through F.D.A. authorization. The panel is made up of scientists with expertise in immunization and public health.