View Winners →
View Winners → 

Governor Gavin Newsom speaks at a press conference Monday. | Screenshot courtesy of California Governor Gavin Newsom on YouTube
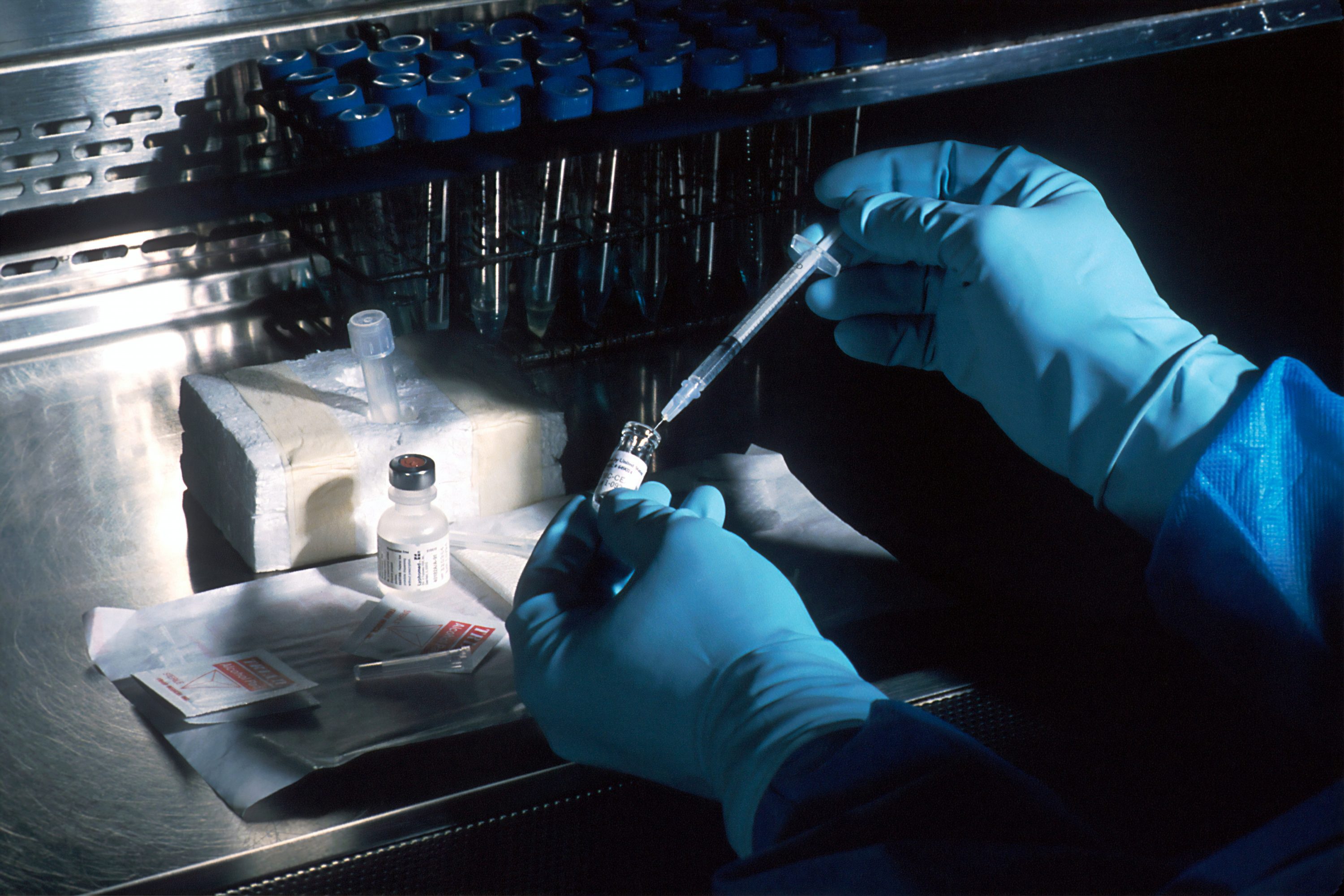
While there is no proven vaccine for COVID-19 yet, a group of scientists will review any vaccine that receives federal approval and verify its safety, before California makes a vaccine available to the public, Governor Gavin Newsom announced Monday.
A group of California physicians and scientists with expertise in immunization and public health have been named to the state’s newly-created COVID-19 Scientific Safety Review Workgroup, which will work with the California Department of Public Health, to “independently review the safety and efficacy” of any vaccine that receives approval from the Food and Drug Administration (F.D.A.) for distribution.
“Of course, we don’t take anyone’s word for it,” Newsom said during a news conference on Monday in which he lauded the state’s experience with mass vaccinations. “We will do our own independently reviewed process with our world-class experts that just happen to live here in the state of California.”
The workgroup is part of the state’s initial COVID-19 vaccine distribution plan, which was submitted to the Centers for Disease Control and Prevention on Friday. California’s vaccination planning process is guided by three principles:
- Ensuring the COVID-19 vaccine meets safety requirements.
- Ensuring the vaccine is distributed and administered equitably, at first to those with the highest risk of becoming infected and spreading COVID-19.
- Ensuring transparency by bringing in community stakeholders from the outset.
Many vaccine candidates are in clinical trials currently, but questions have arisen over whether the Trump administration will try to rush a vaccine to the market. Last month, the president asserted that a vaccine would be available “within weeks” but during a Senate hearing, F.D.A Commissioner Stephen Hahn said that officials would follow guidelines and not cave to political pressure. “Decisions to authorize or approve any such vaccine or therapeutic will be made by the dedicated career staff at F.D.A through our thorough review processes and science will guide our decisions,” Hahn said.
Most experts believe that under a best-case scenario a vaccine will be available by the end of the year or early 2021 but according to the Centers for Disease Control and Prevention, supply will likely be limited at first. “Recognizing that supplies will be limited initially and the first doses of vaccines must go to health care workers, first responders and others who are especially vulnerable to this disease, we are working to ensure that administration and distribution of an approved vaccine is equitable,” Newsom said in a statement released by his office Monday.
While supplies of the vaccine are limited, the state has developed a distribution process that first focuses on healthcare workers, first responders and high-risk groups — people older than 65, those in long-term care and residential facilities, people with disabilities, racial and ethnic minorities, rural populations and those who are incarcerated or detained.
“While a small number of doses of an FDA-approved vaccine could be deployed before year’s end, the reality is that the COVID-19 pandemic will be with us well into 2021 — and widespread vaccine distribution likely won’t occur for many more months,” said Dr. Erica Pan, acting state public health officer.
The state is also planning for challenges with resources related to the distribution of a vaccine such as the supply of needles, syringes, alcohol pads, bandages, masks and personal protective equipment.
The state is also looking at issues with vaccine storage, which requires sterile refrigeration. As a result, the governor warned that dry ice will become a commodity. According to the Associated Press, “Maintaining the cold chain for coronavirus vaccines won’t be easy even in the richest of countries, especially when it comes to those that require ultracold temperatures of around minus 70 degrees Celsius (minus 94 F). Investment in infrastructure and cooling technology lags behind the high-speed leap that vaccine development has taken this year due to the virus.”
Data management is also an area of concern for the state. The California Immunization Registry (CAIR) is the infrastructure currently in place to handle immunization data, but the governor said a team is looking at the efficacy of the technology and at expanding the registry while protecting people’s privacy.
The governor also addressed the need for community education as a new vaccine is introduced. “This vaccine plan will move at the speed of trust,” he said. “You have to have confidence in the efficacy of the vaccine, confidence that we’re not rushing to judgement in terms of its distribution and its accessibility — making sure, again, that we are a third set of eyes or a second set of eyes as is the case here.” The workgroup will be responsible for this outreach which will include trusted messengers, culturally competent information, public service announcements and other educational material.